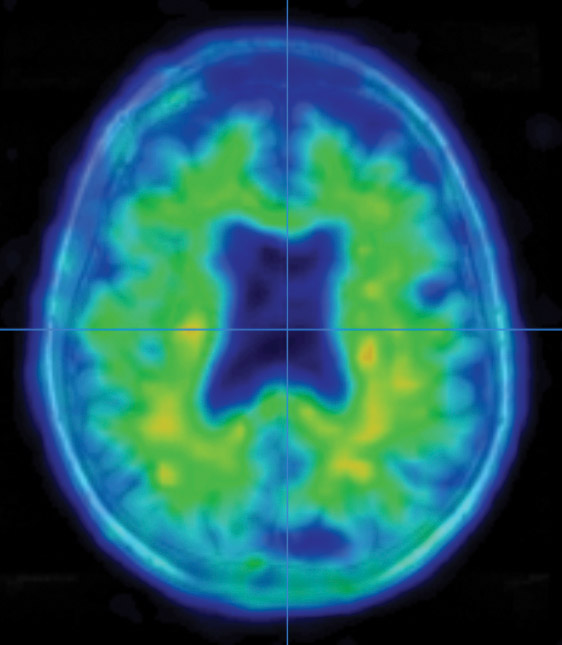
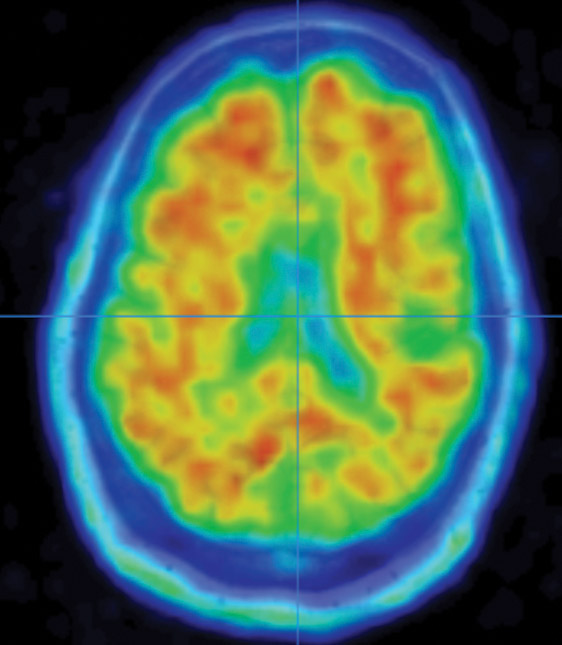
Growing up in the foothills of the Bavarian Alps, Stefan Prokop imagined he would follow in the footsteps of his father, a homicide detective. Prokop’s interest in analyzing evidence and solving mysteries led him to forensic pathology and, during medical school, into the lab of a prominent researcher in Alzheimer’s disease.
“From day one, I was just fascinated by the specific question of Alzheimer’s disease,” he says. “I just walked in and said, ‘That’s what I want to do.’”
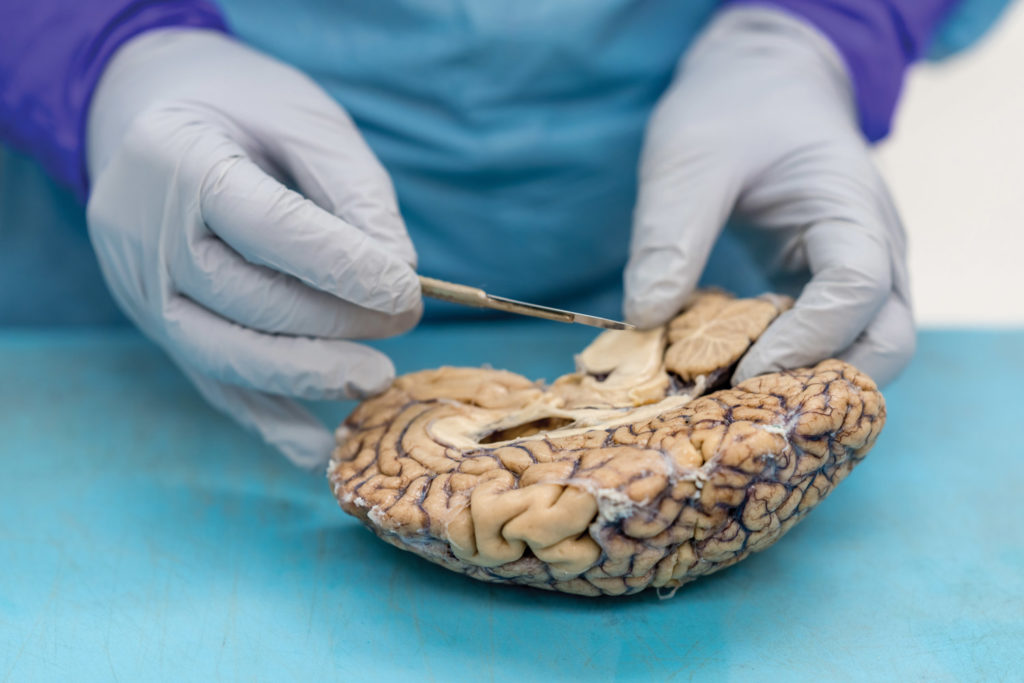
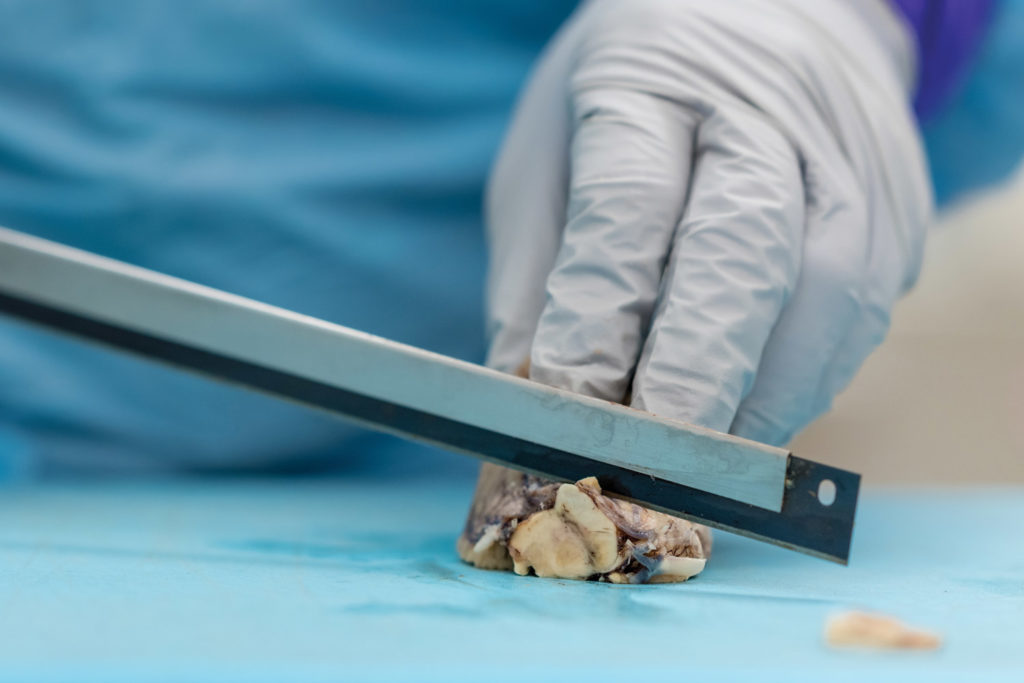
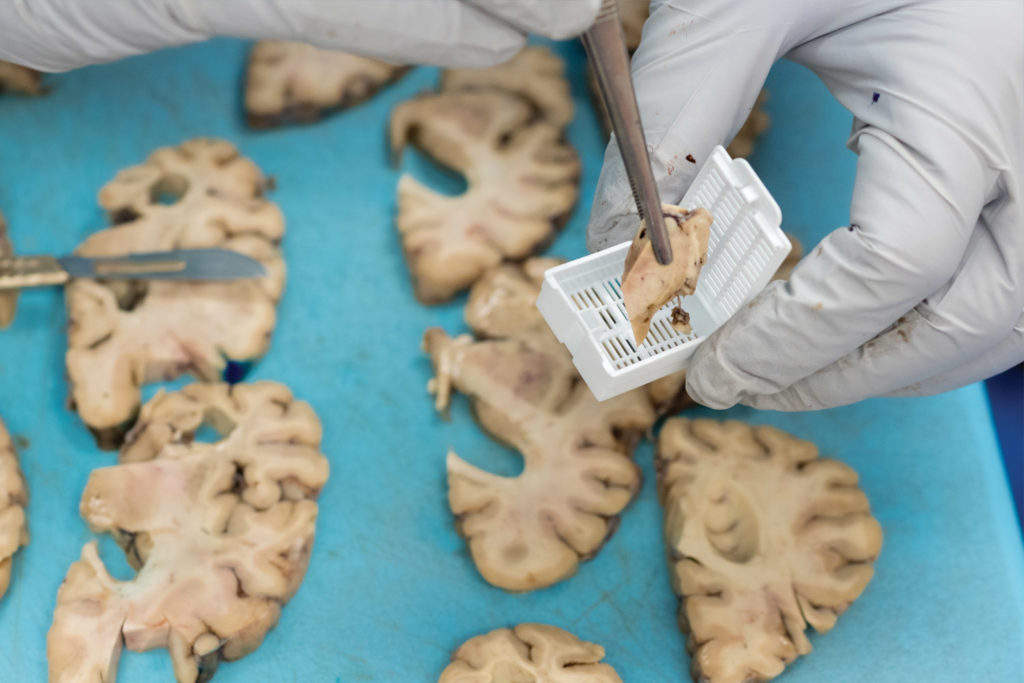
Twenty years later, Prokop stands beneath fluorescent lights in a UF Health lab, a donated brain on the stainless-steel table before him and four medical residents leaning in to see. Now director of the UF Neuromedicine Human Brain and Tissue Bank, Prokop describes telltale signs of Alzheimer’s, such as shrinkage in the frontal lobe and other regions, as he dissects the brain.
A leader in the field of neuropathology, Prokop is among dozens of investigators at the University of Florida hunting for the keys to Alzheimer’s disease, a progressively disabling disorder that afflicts more than 6 million Americans over age 65. That number is projected to grow to 12.7 million by 2050, according to the Alzheimer’s Association. In Florida alone, about 580,000 people currently live with Alzheimer’s, and in just the next five years that number is expected to grow to 720,000.
People with Alzheimer’s slowly lose the ability to handle daily tasks. Beyond memory loss and confusion, they have increasing difficulty with navigation, grocery shopping, bill paying, self-care such as dressing and bathing, motivation, balance and, in late stages, walking and swallowing.
Current treatment options are limited. Some people see temporary improvement in cognitive function and memory using medications, but these do not stop the disease from progressing. The cost in unpaid dementia caregiving by family and friends was valued by the Alzheimer’s Association at $256.7 billion in 2020.
Examining brain segments under a microscope, Prokop seeks to understand which of the molecular and cellular changes related to Alzheimer’s that he sees are a cause of the disease — and which could be a result of it. He is particularly focused on the two main Alzheimer’s-related brain changes: a protein called beta-amyloid that clumps into plaques between neurons and a protein called tau that abnormally accumulates inside neurons to form tangled threads known as neurofibrillary tangles.
Prokop, who was recruited from the University of Pennsylvania in 2019 as the first Fixel Scholar of the new Norman Fixel Institute for Neurological Diseases at UF Health, is investigating how these plaques and tangles interact and damage the brain.
Like a good detective, he is coming at it from multiple directions.
Under a new National Institutes of Health grant, he and UF neuroscientists Todd Golde and Yona Levites are exploring a large number of proteins believed to accumulate alongside amyloid — looking simultaneously at their role in donated human brains and mouse models to reveal any possible correlation with severity of Alzheimer’s symptoms. Early data suggest many of these could be key players in Alzheimer’s disease and potential new targets for intervention.
Prokop is also collaborating with UF neurogeneticist Matt Farrer to sequence the genomes of donated brains. This could help identify protective gene variants, for example in a person who had significant Alzheimer’s pathology at autopsy but never had dementia in life.
To Prokop, one way to better understand Alzheimer’s is to examine brains from diverse racial backgrounds, as most published research on the disease involves white people of European ancestry. In his role as leader of the neuropathology core of the 1Florida Alzheimer’s Disease Research Center — a UF-led consortium of top research institutions known as the 1Florida ADRC — he is working to spread the word and encourage people of diverse racial backgrounds to make arrangements for future brain donation.
“In the end, we want to treat the population,” Prokop says. “We don’t want to treat one subgroup of the population, so what we hope to gain from our research is better insight: Are these genetically diverse populations similar, or have we studied one type of Alzheimer’s disease that occurs in Caucasians, and is the disease different?”
The 1Florida ADRC, funded by the National Institute on Aging, enrolls research participants with and without memory disorders and tracks them for years to reveal factors that influence progression rates, using blood samples, cognitive testing and state-of-the-art brain imaging. Some enrollees also participate in clinical trials to test potential new medicines.
One focus that distinguishes the 1Florida ADRC from 32 other such Alzheimer’s Disease Research Centers in the country is that over 60% of participants are Hispanic.
“We’re hoping to make a major contribution to understanding Alzheimer’s disease by understanding how it impacts diverse populations differently,” says Golde, director of UF’s McKnight Brain Institute and principal investigator of the 1Florida ADRC, which is a collaboration of over 40 researchers from UF, Mount Sinai Medical Center in Miami Beach, the University of Miami, Florida Atlantic University and Florida International University.
The clinical arm of the 1Florida ADRC is tracking up to 600 study participants, who are undergoing testing in a five-year, $15 million project.
Artificial intelligence will be used to combine all the data — behavioral, clinical, biological and advanced neuroimaging biomarkers — to predict future development of Alzheimer’s, says UF neuroscientist David Vaillancourt, who leads the biomarker core for the study. The data will be available to Alzheimer’s researchers nationwide.
The focus on diversity extends to those who study the disease. Valerie Joers and Karina Alviña are the first two scholars selected for the new “AlzSTARS” program, which recruits and trains junior investigators from diverse racial, ethnic and gender backgrounds, as well as from diverse locations and research interests.
Humbled, but Optimistic
Golde, an internationally known expert in the scientific understanding of Alzheimer’s, describes the field’s 30-year pursuit as “humbling.” Initial excitement over the first discoveries of Alzheimer’s-related brain changes led to many trials of therapies that, despite sound scientific basis, have failed to alter the course of the disease. But with incremental successes in the lab, recent increases in federal and state funding, the passion of up-and-coming young researchers and knowledge gained from past failures, he sees a future in which Alzheimer’s can be both treated and prevented.
“I’m optimistic that we’re going to get there, but it’s going to take some time,” he says. “It’s not going to be necessarily a linear path with one success after another. But we have learned enough from our failures that we’re going to have new approaches.”
One place where those new approaches are evident is at UF’s Center for Translational Research in Neurodegenerative Disease, where neuroscientists are creating new tools, like unique mouse models.
“At UF, we have some of the best basic science modelers, who have devised mouse models that are widely used throughout the world,” says Steven T. DeKosky, a renowned neurologist honored in 2020 with the Alzheimer’s Association’s Henry Wisniewski Lifetime Achievement Award.
“These models are yielding new insights into one of the big unanswered questions: How does beta-amyloid talk to tau?” Golde adds. “Current thinking is that amyloid pathology precedes tau in neurodegeneration and nobody understands the link, but we think that these novel mouse models are beginning to more faithfully reproduce that crosstalk and may give us insights.”
UF neuroscientist Jada Lewis, in collaboration with investigators at the University of Minnesota and Harvard, has developed mice that express human tau that can be turned on and off like a light switch using the antibiotic doxycycline. This model has been cited in over 1,300 research papers, according to the database Web of Science.
“Mouse models are yet another tool in the arsenal of fighting against Alzheimer’s disease,” says Lewis, deputy director of the McKnight Brain Institute. “We exhaust every possibility we can through cell models, because it preserves animal use and it’s quicker and often cheaper. But there are some things you can’t model in cell culture or on the computer, and that’s the actual interaction between tau and amyloid pathologies and resultant behavioral problems.”
With funding from the National Institute on Aging and the National Institute of Neurological Disorders and Stroke, Lewis is working with fellow UF neuroscientist David Borchelt to produce new strains of mice that model the amyloid and tau pathologies characteristic of human Alzheimer’s disease. Their newest models should significantly reduce the number of mice that researchers have to use in many of these complex studies, Lewis says.
“In general, the field thinks that amyloid comes first and then tau is downstream of that,” Lewis says. “But there’s also another school of thought that tau may come first and interact with amyloid in that manner.”
Pinpointing the timing could be instrumental in developing new treatments that interrupt the disease.
Lewis and Borchelt are investigating what happens when tau pathology is turned on first and, conversely, what happens when amyloid pathology is turned on first. They seek to answer the question: How does the timing affect the formation of global amyloid pathology often seen in Alzheimer’s disease patients?
“We know there’s a connection between the amyloid pathology and the tau pathology,” says Borchelt. “If you have amyloid, tau pathology seems to be worse — more severe or perhaps in different locations. What we’re trying to understand is the connection between those two.”
In addition to modeling, Lewis’ other main focus is on training the next generation of researchers who will take on the complexity of Alzheimer’s and related dementias. She and two other UF scientists lead an NIH program to train postdoctoral fellows in clinical and translational research in Alzheimer’s disease.
“The more people we get looking at it in different directions,” she says, “the better we’ll be able to describe the disease and attack the disease.”
Beyond Amyloid and Tau
Among those looking at Alzheimer’s from a different perspective is Malú Gámez Tansey, a prominent neuroscientist recruited to UF from Emory University in 2019 through the founding gift for the Norman Fixel Institute.
Tansey’s research focuses on the roles of inflammation and the immune system in the development and progression of neurodegenerative diseases, including Alzheimer’s. As humans age, she explains, our immune systems are less efficient and more vulnerable to potential exposures, leading in some cases to chronic inflammation as toxic proteins accumulate and aren’t cleared from neurons.
“We believe that could be a result of aging, sluggish or nonfunctional immune cells,” says Tansey, co-director of the Center for Translational Research in Neurodegenerative Disease. “The vacuum cleaners of the brain, the vacuum cleaners of your organs that keep the house tidy, become less competent as you get older.”
Meaning, she says, amyloid buildup could be a consequence, rather than a cause, of Alzheimer’s.
Tansey believes Alzheimer’s and other dementias are influenced by both genetics and environment, including variables such as diet, physical exercise, quality and quantity of sleep, and exposure to pesticides and pathogens. “We know that a small percentage of cases for Alzheimer’s and related dementias are strictly genetic — maybe 5 to 10%,” she says.
In one line of research in her lab, a high-fat, high-sugar diet resulted in an acceleration of amyloid buildup and immune dysfunction in mice genetically engineered for Alzheimer’s pathology.
“The high-fat, high-fructose diet made everything worse,” Tansey says. “It accelerated the whole timeline, which suggests that yes, having chronic inflammation in a setting where you are genetically predisposed is a bad thing.”
Her lab then tested an experimental drug on mice on a high-fat, high-fructose diet or a control diet. The drug, XPro1595, is designed to reduce neuroinflammation. By selectively inhibiting an inflammation-causing protein called soluble tumor necrosis factor, or soluble TNF, in the gut and brain, the drug “was able to reverse some of those changes in the stool microbiome,” she says, referring to changes in bacteria and other microorganisms of the gut. “We thought this was super interesting and may be important.”
It is a critical step showing that some immune system-targeted interventions have the potential to mitigate inflammation and prevent worsening, even when genetic predisposition increases risk, Tansey says.
XPro1595, or pegipanermin, was co-invented by Tansey before coming to UF, and it has been tested in a small, early clinical trial in Australia led by the biotech company INmune Bio, for which Tansey is a consultant. She also owns stock in INmune Bio. The trial was partially funded by a “Part the Cloud” award from the Alzheimer’s Association. In the trial, the drug lowered brain inflammation, decreased nerve cell death and improved synaptic function. The trial did not evaluate cognition. The next step will be a double-blind, multisite Phase 2 trial to determine if control of neuroinflammation could have an impact on cognition.
Superagers
To better understand why some people get Alzheimer’s disease, Glenn Smith and Steve Anton are studying those who manage to avoid it.
The two clinical psychology researchers are co-principal investigators of the “Over 90 Study,” which examines those who have remained cognitively healthy, despite in some cases having a genetic predisposition for Alzheimer’s.
The hope is that by revealing the secrets of so-called “superagers,” researchers can learn how to prevent Alzheimer’s in others.
“Ten to 15% of people who die with Alzheimer’s disease changes in their brain never developed dementia,” Smith says. “So how did they escape the cognitive effects of that pathology?”

To launch the Over 90 Study, a team of 10 UF Health experts worked together to determine a definition for successful aging that could be assessed from a medical record, such as no history of stroke or diagnosis of dementia and living independently, among other factors.
The team then ran an algorithm to tally such patients in the UF Health-based OneFlorida+ Data Trust, a repository holding millions of anonymous electronic medical records.
With de-identified medical record information, researchers may identify a cohort of patients who meet certain criteria, but they are not able to identify any specific individual. Then, under a process regulated by the UF Privacy Office and UF Institutional Review Board, which protects the rights of human research subjects, postcards were sent to potential study participants in Alachua County, and over 200 people responded and participated in a telephone screening, mental status test and saliva test for genetics.
That first stage of the study aimed to demonstrate that medical records could be used to create a large enough study set, and the results were encouraging. “We’ve successfully enrolled over 100 older individuals at this point, and we’re continuing to recruit,” Anton says.
Smith and Anton have collaborated with Farrer, the neurogeneticist, who has completely sequenced the whole genomes of the first 20 participants to see what is revealed. They’re also recruiting a second group of 85- to 90-year-olds using the same criteria.
The next step is to work with partners at other institutions to design a large-scale, multisite trial that would enroll 5,000 successful agers from across the country, including over 2,000 individuals who identify as African-American or Hispanic.
“What we’re trying to figure out,” Smith says, “is are there both genetic and lifestyle factors that protect people against cognitive decline? So, we first need to figure out who are the people who avoided it. And then we could envision, in addition to collecting biological samples and looking for genetics, subsequently doing interviews to try and map out what aspects of physical activity and social engagement might distinguish these people. What things did they avoid?”
One thing is known for certain.
“People over age 90 are rare,” Smith says, “and people over 90 who have aged successfully are rarer still.”
Which is why the Over 90 Study has one final goal: to determine if there are specific activities — cognitive, physical or otherwise — that could help these superagers stay that way.

Sources:
Stefan Prokop, M.D.
Director, UF Neuromedicine Human Brain and Tissue Bank
sprokop@ufl.edu
Todd Golde, M.D., Ph.D.
Director, Evelyn F. and William L. McKnight Brain Institute
tgolde@ufl.edu
David Borchelt, Ph.D.
Professor of Neuroscience
drb1@ufl.edu
Malú Gámez Tansey, Ph.D.
Co-Director, Center for Translational Research in Neurodegenerative Disease
mgtansey@ufl.edu
Glenn Smith, Ph.D.
Professor of Clinical and Health Psychology
glennsmith@phhp.ufl.edu
Jada Lewis, Ph.D.
Deputy Director, McKnight Brain Institute
jada.lewis@ufl.edu
Yona Levites, Ph.D.
Research Associate Professor of Neuroscience
levites.yona@ufl.edu
Brain Check
UF leading movement to check for cognitive impairment before surgery
By Michelle Koidin Jaffee

It’s routine for patients with certain heart conditions to have their cardiac function checked before surgery for a non-heart-related issue, such as a knee operation. But it’s not routine for certain patients who are at risk of cognitive complications to have a presurgery brain check.
UF neuropsychologist Catherine Price wants to change that.
In 2017, Price founded a first-of-its-kind clinic at UF Health Shands Hospital that provides presurgery screening for adults over age 65 to detect any form of cognitive impairment, from mild impairment to more severe forms such as Alzheimer’s disease or other dementias. Four years later, more than 1,000 patients a year go through screening at the clinic, called the Perioperative Cognitive Anesthesia Network.
Although adults over 65 commonly undergo surgery for urgent medical reasons as well as quality-of-life ones like joint replacement, there is a lack of evidence-based, perioperative medical care in older adults with cognitive impairment, Price says.
It’s estimated that 20% of seniors preparing for surgery have preexisting signs of neurocognitive disorders, research by Price and others shows, and such underlying conditions elevate the risk of complications like delirium. Delirium is an acute state of confusion and disrupted attention that is associated with longer hospital stays.
“We need to identify vulnerable people before surgery,” she says. “There needs to be a change in the way clinicians adjust patient care based on brain profiles. People with neurodegenerative disease risk factors and diagnoses require special consideration when it comes to anesthesia and surgery procedure choices.”
The Perioperative Cognitive Anesthesia Network team includes anesthesiologists, neuroscientists, neurologists, surgeons, biomedical engineers and experts in artificial intelligence, and it has clinical, research and training goals. Funded by the National Institutes of Health, it is considered a model nationwide, says UF anesthesiologist Patrick Tighe, co-director of the program.
“Now, if you go to the anesthesiology national meetings, presurgical cognition is a hot topic,” Tighe says. At a pre-pandemic American Society of Anesthesiologists general assembly, he says, Price’s work was held up as “the idealized example so far of how to do presurgical cognitive assessments.”
Under the program, cognitive assessment begins with drawing an analog clock using a high-tech pen equipped with a tiny camera that captures images 80 times per second. The image is analyzed by neuropsychologists to determine if further cognitive testing and neuroimaging scans are needed.
The clinic, also led by neuropsychologist Kristin Hamlet, then produces a report to inform anesthesiologists, surgeons, geriatricians and primary care doctors about a patient’s cognitive status and provides personalized recommendations, such as use of a special monitor that assesses the depth of sedation during surgery or adjusting the dosage of certain drugs used for sedation or nausea.
Under ongoing research, the clock drawing is also run through an artificial intelligence algorithm as a tool to make
a prediction of cognitive outcomes,
Tighe says.
A next important step, says Price, is a large, multisite study using artificial intelligence to analyze medical records to predict outcomes for people with preexisting dementia who undergo surgery.
Sources:
Catherine Price, Ph.D.
Director, Perioperative Cognitive Anesthesia Network
cep23@phhp.ufl.edu
Patrick Tighe, M.D., M.S.
Co-director, Perioperative Cognitive Anesthesia Network
ptighe@anest.ufl.edu
Kristin Hamlet, Ph.D.
Clinical Assistant Professor of Psychology
kmoffett@phhp.ufl.edu