In January 2020, rumors of a nascent disease proliferating through Wuhan, China were spreading. Tom Hladish, a quantitative epidemiologist at the University of Florida, tuned in to a discussion among epidemiologists attempting to predict how the drama would unfold.
“I was in South Africa with some other epidemiologists. They were trying to do back of the envelope calculations.” Hladish said. “And I remember asking them, what exactly is going on with this?”
Hladish, a member of UF’s Emerging Pathogens Institute, has spent the past two and a half years developing forecasting models to understand and predict the evolving dynamics of SARS-COV-2. His team has accurately predicted peaks of COVID-19 waves in Florida and his advice has guided decisions made by public health officials and hospital administrators. However, accurately modeling the virus has presented unparalleled challenges.
Early in the pandemic, state officials asked Hladish, with 30 minutes notice, to provide week-by-week predictions of hospitalization burden in all 67 Florida counties for a 6-month time horizon.
“I didn’t expect the state to initially turn to me, with no knowledge of what interventions they might be able to try. That was extraordinarily stressful,” Hladish said.
Despite the virus’s mysteries and stressors, Hladish had the intuition, team and toolkit to digest the deluge of COVID-19 information. When little was known about disease transmission, he used branching process models to predict the first wave’s early growth phase – the period when almost everyone an infected person encounters is susceptible. With an estimated basic reproduction number (R0) of 3 for COVID-19, which means an infected person on average infects three other people, infections tripled in size based on the generation time – the average time between when an individual is infected and when they infect others. For COVID-19 that was roughly between five to six days.
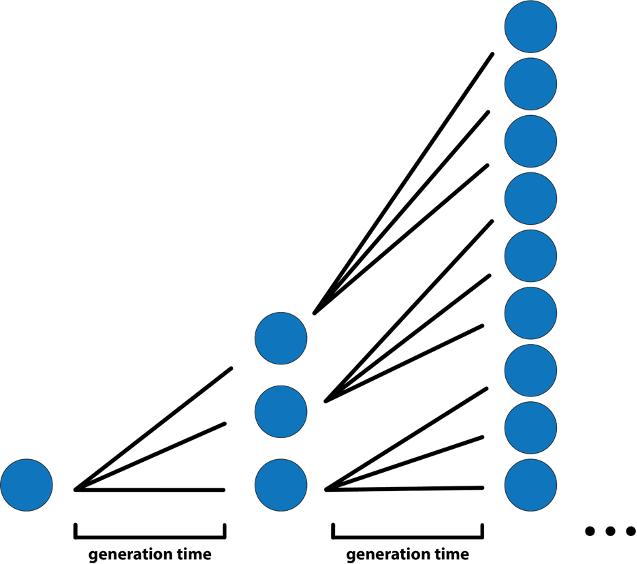
“The less you know, the simpler the model has to be.” Hladish said.
As weeks became months and knowledge of the virus became increasingly complex, Hladish and his team analyzed state data to construct a more detailed and empirically derived agent-based model – a computational model that can simulate the interactions of individuals (agents) with their environment and each other. In this more realistic model, individuals could go to work, visit friends and patronize businesses. This model allowed Hladish to predict the omicron variant’s increased magnitude of infections compared to delta in Florida.
Regardless of these advancements, keeping up with COVID-19 has been a marathon. The emergence of variants such as alpha, delta and omicron required Hladish and his lab to quickly revise code in the model to account for changing viral characteristics, including infectiousness, virulence and incubation period. Now, Hladish can run simulations where individuals are infected with specific variants.
“I’m kind of glad that we were pushed to go there, because it’s a way cooler model.” Hladish said. “It sets up the groundwork for really important questions about the spread of new variants, and potentially even how they arise in the first place.”
Throughout the pandemic, Hladish never felt like he was doing enough. However, in ceaseless COVID-19 conversations, he became enamored with explaining science to people. Journalists, public health authorities, hospital staff, and anyone he spoke to leaned on him to interpret models and quell the rumors.
Epidemiologists like Hladish are crucial in demystifying perplexing pandemic patterns, but the science of quantitative epidemiology has largely operated independently of public health. To continue guiding decision making, models need to be debugged and well-understood. As new COVID-19 variants are introduced, forecasting their impact with improved models would allow public health officials to make more informed and swift decisions, Hladish says.
“The pandemic has been a wake-up call for epidemiologists to be more embedded in public health,” Hladish said. “We’re in a position now where we can internalize the right lessons or the wrong lessons. And it will determine how the next pandemic plays out.”
-Andrea Tamayo


