A group of UF researchers, students and lab technicians across campus pulled together in late March to assemble a high capacity COVID-19 testing lab in just 10 days, to help fill an acute gap in testing capacity in the region. Under normal circumstances, it would take four to six weeks to create this type of specialized laboratory which is capable of processing large test volumes.
But these are no longer normal times, and the group was fueled by an urgency that their skills and knowledge could help in the fight against a virus that threatens the state’s health and security.
The effort began when a research project on community surveillance of the viral disease known as COVID-19 widened in scope to include testing at The Villages®, Florida’s largest retirement community. A collaborative effort with UF Health made drive-through testing available to community residents who met the CDC criteria for testing, while those who did not meet the criteria had the choice to enroll in EPI’s study and still receive a COVID-19 test. Retirees comprise a particularly vulnerable group because people ages 65 and older are at higher risk for developing severe outcomes from COVID-19.

“We are still learning how this virus is transmitted,” says EPI Director J. Glenn Morris. “And, in developing appropriate prevention strategies for communities such as The Villages, it was important to know how frequently people within the community were infected, but without symptoms. Community surveillance of this type provides a way to protect the general public by identifying and isolating people who may be spreading a disease unknowingly.”
The only catch? The research lab processing the tests at the Emerging Pathogens Institute would have to scale up from running a few dozen tests per day to 1,300 per week.
Safeguarding our community
Morris’s community surveillance vision grew legs when it met up with UF virologist and EPI researcher John Lednicky, who is also a professor in UF’s College of Public Health and Health Professions, department of environmental and global health. A few years ago, Lednicky and Morris paired up with UF Institute of Food and Agricultural Sciences assistant professor John Driver to determine whether Brazilian free-tailed bats living in and around UF’s campus carried coronaviruses.
By analyzing the bats’ feces, the investigators could look for evidence of coronaviruses carried by bats without having to handle or trap live animals. The project arose from Lednicky’s curiosity about animal viruses that may cause respiratory illnesses of unknown origins in Florida.
For this study, Lednicky developed two tests: one for alphacoronaviruses, the other for betacoronaviruses. (See EPI Explainer.) The tests were specifically designed to detect bat viruses, and did not detect common human coronaviruses. By studying viruses shed in the bats’ feces, the team found evidence that the bats carried a new alphacoronavirus, but did not find evidence of a new betacoronavirus.
“I put it in my freezer, and forgot about it,” Lednicky says of the test. “It sat there for the last few years. But it turns out it’s 100 percent compatible for this new virus. And the reason it works so well is because this new virus likely came from bats too, and it’s also a coronavirus.”
When COVID-19 appeared, Morris adds: “We were able to take advantage of this work to quickly design a PCR-based test for this new virus. The test that we designed uses an approach similar to that used by European investigators, and provides an alternative to the test developed by the CDC.”
Although the EPI houses the largest collection of biosafety level III labs south of the U.S. Centers for Disease Control, it is a designated research facility and it is not certified to perform clinical tests.
“Still, we want to offer our expertise in this area,” Morris says. “And John’s test, run in parallel with the CDC test, offered the perfect opportunity for us to contribute an understanding of how this novel virus might be spreading within our communities.”
But what began as a small research effort to offer tests to concerned students and area medical professionals soon blossomed as the opportunity arose to test hundreds if not thousands of Floridians thanks to UF Health’s initiative to screen retirees at The Villages. While people who met the CDC’s testing criteria were offered a clinical test, those who did not meet the testing guidelines and those who were simply concerned, were offered the chance to enroll in EPI’s community surveillance research.
Lednicky’s lab manager, Julia Gibson, reflects on what this meant: “In a research lab, we rarely run more than 12 to 20 tests a day for anything. Suddenly, we had to figure out how to process up to 400 tests a day.”
Her team had been running tests by hand, but then, “We needed a robot,” she says.
Assembling the lab
Gibson and Lednicky had previously created an equipment dream list after brainstorming what would be needed to expand the scope of testing within their laboratory. One of the items on the list, Gibson said, was an Eppendorf epMotion robot. This is an automated pipetting machine for handling large numbers of liquid samples, in this case up to 96 samples in a single batch.
Gibson weighed a few other robot options but decided to go with her first choice because of its flexibility to be programmed for test kits produced by a variety of manufacturers. Plus, she’d already received training on it and felt she could hit the ground running. This was followed by a telephone call from Dr. Morris to Adrian Tyndall, M.D., Dean of the UF College of Medicine, explaining the need and asking if the College of Medicine could help provide support for the purchase of this critical piece of equipment. With Dr. Tyndall’s approval, the group was in business.

Acting as lab foreman, Gibson undertook the daunting task of figuring out what other equipment and supplies would be needed to drastically scale up their operation.
Meanwhile, Morris asked EPI faculty members Tony Maurelli, Tara Sabo-Atwood and Joe Bisesi —all professors in UF’s College of Public Health and Health Professions, department of environmental and global health — to supervise recruiting lab technicians, post-doctorates, and graduate students across campus to work specific lab stations. While Gibson coordinated the equipment needs, the faculty scouted for skilled lab workers.
“We did not have enough time to train people from scratch,” Maurelli said. “But we could train people with the right skill set to run our protocols.” The group contacted colleagues and asked for recommendations for lab personnel with very specific skills and training.

Within a few days, the coalition of volunteers grew from an initial core of three to 15.
“But we had to also weigh that processing these tests was a potentially exhausting work schedule, and that this could go on indefinitely,” Maurelli added. “We structured it in shifts, and then recruited backups for each shift member so that no one would have to work when fatigued.”
Meanwhile, the lab supplies and equipment ordered by Gibson were delivered to the EPI building and she found herself negotiating for space. One researcher with an active project underway volunteered to give up coveted bench space adjacent to the facility’s flex labs, which feature negative pressure and biosafety cabinets. This meant that the new high capacity lab activities could be condensed to one building floor. The sacrifice was key to making the new lab’s workflow efficient, Gibson says.
Amid the deliveries and spatial reconfiguring, Gibson coordinated biosafety cabinets that needed recertifying and drafting multiple standard operating procedures.
“The amount of time it took to research, order and assemble the equipment was enormous. It was maddening,” Lednicky says. “Gibson did the leg work to make it possible. Without her, this would not have happened in the timeframe it did.”

The robot was delivered on March 20th. The next day, the newly-assembled team trained. Two days later they began receiving up to 300 tests per day from participants enrolled in their study from The Villages. Participants’ results were ready within 48 hours, and this was soon shortened to 24 hours as the team became more efficient.
“We kept moving back and forth between different levels of chaos,” Maurelli says. “It was a real team effort. We had a variety of different people who came in with a lot of different skill sets that we were able to use. We broke it down the testing process piece by piece into what needed to be done, and then trained people for each task.”
Behind the scenes
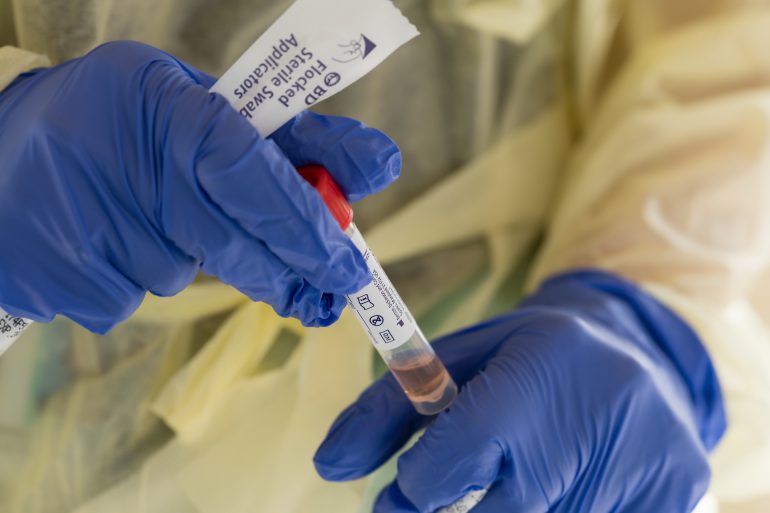
When a nasal swab sample is collected from a study participant, it is stored in “viral transport medium” in a collection tube. This medium, Lednicky’s “special recipe,” is made at UF-EPI. It keeps viruses alive so that his lab can isolate and grow them for research purposes. Some of Maurelli’s lab members are contributing to the testing efforts by concocting large quantities of this medium.

When the samples arrive at the EPI, the collection tube exteriors are disinfected to protect the lab workers who will handle them. A portion of each sample is transferred to a new analysis tube. The remainder in the original collection tube is stored frozen for additional tests. Next, a lysing buffer agent is added to the analysis tubes to inactivate any virus that might be in the viral transport medium. The lysing buffer releases genetic material from the virus in the form of nucleic acid.

After the samples are lysed, the genetic material is extracted and purified. And this is where the robot saves time and scales up capacity.
“We have to extract the nucleic acid, the RNA, away from all the other stuff that is there,” Maurelli explains. “And we need to purify the RNA and remove all the other things which may interfere with the reactions. That is what the robot does, and this is the big advantage of the high throughput capacity. It’s a tedious set of steps to extract and purify, but the robot can do it very efficiently in this multiple well format.”

After the RNA is extracted and purified it then goes to a PCR station where the first step is to reverse transcribe the RNA into a synthesized strand of complimentary DNA and then perform a polymerase chain reaction test which amplifies the genetic material to a measurable quantity. This is the part of the process that verifies the presence or absence of viral gene sequences specific to the novel coronavirus now known as SARS-CoV-2, which causes COVID-19.
For now, the research study uses CDC test kits alongside Lednicky’s test, which looks for different targets, to validate the sensitivity of his test and evaluate if it can be used on its own. If it can, this will free up resources to devote to research without having to compete for limited tests which are also needed for clinical cases.

Next steps
Although the study continues to offer research testing to residents of The Villages, the EPI is already moving forward to offer testing to other community groups. Testing has been initiated for specific homeless populations in Gainesville, and a program for free testing of Emergency Medical Services personnel in Alachua County is now underway, as is a testing program in Jacksonville.
The groups being tested, including the homeless and EMS professionals, may seem like disparate populations at first glance, but they are all uniquely vulnerable either through risk factors such as older age, direct occupational exposure, or lack of access to care and increased hygiene measures such as hand washing.
“We can’t ignore these vulnerable populations in our own community, and in our region” Maurelli says. “I am proud that the folks here at UF are leading the way in assessing ways in which this virus is transmitted, within the groups being studied. Results from these studies should have an impact on public health interventions at local, regional, and national levels.”

EPI EXPLAINER: HOW MANY KINDS OF CORONAVIRUSES ARE THERE?
Coronaviruses are divided into four major groups: alpha coronaviruses, beta coronaviruses, delta coronaviruses and gamma coronaviruses. Human coronavirus stains 229E and NL63 are in the alpha group. Human coronavirus OC43, the new SARS CoV-2, and the viruses that cause Severe Acute Respiratory Syndrome (SARS) and Middle Eastern Respiratory Syndrome (MERS), all belong to the beta group. The delta group contains viruses that affect birds and pigs, and the gamma group contains viruses that affect birds and beluga whales. For more on the types that affect people, visit the CDC.
This article originally appeared on UF EPI News.