A University of Florida research team is harnessing the power of genomic editing to illuminate druggable targets in human cells for the fight against COVID-19.
CRISPR Genome Editing
By taking advantage of high-containment labs in UF’s Emerging Pathogens Institute—especially designed for studying highly contagious and virulent pathogens—the team is using CRISPR genome editing techniques to screen human cell lines. Their goal is to discover genetic factors that either hasten or thwart infection by SARS-CoV-2, the virus which causes COVID-19.
The interdisciplinary team includes Christopher Vulpe M.D., a professor in UF’s College of Veterinary Medicine, in collaboration with Stephanie Karst, a professor in UF’s College of Medicine, and Mike Norris, a professor in UF’s College of Liberal Arts and Sciences. Karst and Norris are EPI faculty.
“While I know squat about viral pathogens, my group has used CRISPR a bit for toxicology, although not for studying viruses,” Vulpe says. “Luckily, some colleagues with deeper experience in studying viruses were thinking along the same lines, that we should apply genome editing techniques to answer questions about what the virus needs from its host.”
Karst was already working on a National Institutes of Health-funded project to identify host factors for other viruses when she contacted EPI Director Glenn Morris, M.D., about working on COVID-19 research. The virulent SARS-CoV-2 virus must be studied within a special type of lab; and the EPI houses the highest concentration of these on UF’s campus. Morris connected Karst and Vulpe, and also brought in Norris, who has an extensive background working in biosafety level III and high containment labs.
The trio recently started their project with $89,000 in seed funding from UF’s Clinical and Translational Science Institute. The funding is a portion of a National Institutes of Health grant, Creating the Healthiest Generation, which is repurposed to support projects internal to UF.
Investigation goals
The team has two main goals, the first of which is to learn what the virus needs from its host to make copies of itself, a process known as replication.
“Viruses steal what they need,” Vulpe explained. “They can’t make more of themselves without a host. If the proteins that the virus is trying to use are not there, then the virus doesn’t replicate, and the host doesn’t support infection. Using CRISPR is a way for us to identify what genes and proteins are important for a SARS virus to infect a human cell.”
The second goal is to understand better how the host cell responds to the viral infection to protect itself. Using CRISPR can also allow researchers to uncover specific host factors that block viral replication. Host factors that are required for viral infection, or block it, both represent candidate drug targets.
Screening for survivors
Prior research by others established that CRISPR techniques are useful for studying interactions between hosts and viruses. The UF team is using “libraries” of human cells, which have been modified by CRISPR gene editing techniques to either knock out specific genes or amplify the expression of their proteins.
In the beginning, the team was not sure they would find a commercially available cell line that would work. But by overexpressing ACE2, the receptor to which the virus binds to unlock entry into the cell, the researchers made quick headway.
“We were very excited to learn early on that when exposed to the virus, the cell line HEK293T—which we manipulated to overexpress ACE2—experienced full cytopathic effect,” Karst says.
The phrase cytopathic effect refers to cellular death. In other words, all the cells in their sample were killed, or lysed, by the virus—a necessary outcome to identify genetic editing that allow for survivors.

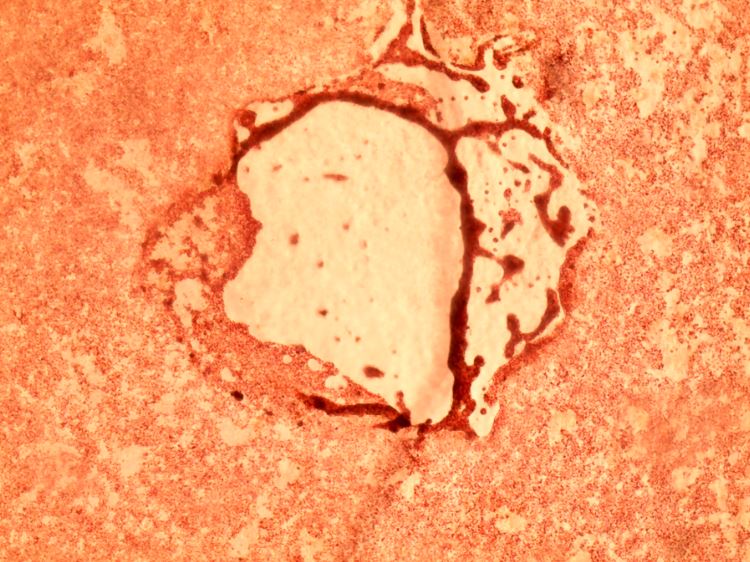
In these images, the lighter-colored voids in the middle show where SARS-CoV-2 virus has killed a thin layer of live, red-hued African green monkey cells (left) and human kidney cells (right). The virus enters a cell, hijacks cellular machinery to make copies of itself, then causes the cell to rupture so that its viral progeny are released to infect neighboring cells. As the process repeats, plaques of killed cells form. (These images are courtesy of the study authors.)
“We are looking for both host restriction factors, and host replication factors,” Karst says, referring to genetic factors that either restrict the virus from replicating, or contribute to its growth.
To do this, the team will use a library of single-guide RNAs which ride on a lentivirus, which acts sort of like an Uber driver, to their gene target. Once there, the guides point the Cas9 enzyme to its target where it then knocks out specific genes or causes them to overexpress proteins. These modified test cells—each edited to manipulate one of 20,000 gene targets, are then pooled together and treated with the SARS-CoV-2 virus.
Cells that survive this treatment will be evaluated to identify genes or proteins that promote cell survival against SARS-CoV-2. These factors will then become targets for the discovery of new drugs. Ideally, existing FDA-approved drugs could be screened for activity against these newly identified genetic targets. But if there is not an existing drug, researches could begin work to develop a new one.
Up next
Karst also plans to identify and characterize virulence factors with a broad look at the coronavirus family. Before the world met SARS-CoV-2, there was a prior version that emerged in China’s Guangdong province in 2002. Called SARS-CoV, it was more deadly but spread less easily than today’s second version. Both SARS pathogens are in the same virus family that includes human coronavirus NL63, which is a strain that causes common colds. The connection is perplexing to researchers such as Karst.
“Why is it that some less deadly coronaviruses, such as NL63, use the same ACE2 receptor but produce only mild illness?” Karst asks. “Whereas SARS-I and SARS-II produce severe disease despite using the same cellular front door to gain entry to a host?”
Karst plans to use their data to leverage the pilot study into a new grant that will investigate coronavirus virulence factors. If researchers can gain a better understanding of virulence factors, it could help in the race to design therapeutic drugs.
This story originally appeared on UF Emerging Pathogens Institute.
Check out more stories about UF research on COVID-19.